Stabilization Using Membranes
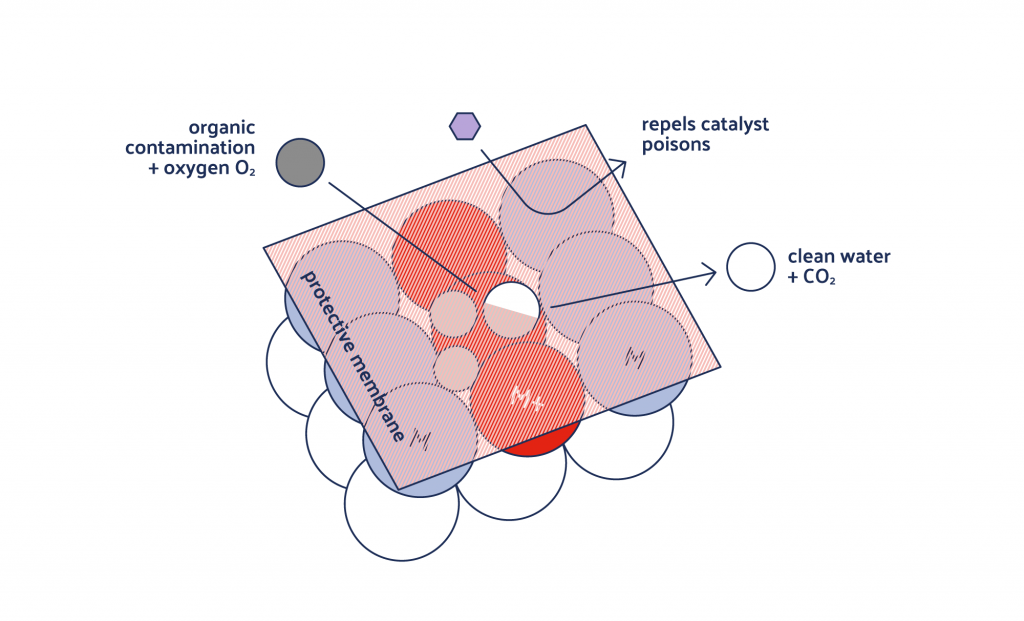
How do we achieve the necessary stabilization? With our very own semipermeable membrane. This membrane is based on crystalline compounds of group 11 metals which are thermodynamically stable in aqueous media.
Its microporous structure allows oxygen to pass through, but it remains impermeable to catalyst poisons such as sulfur compounds. Thus, the active area beneath stays permanently protected – even in aqueous media. And we are not talking days here! We are talking about a service life of months, even years.
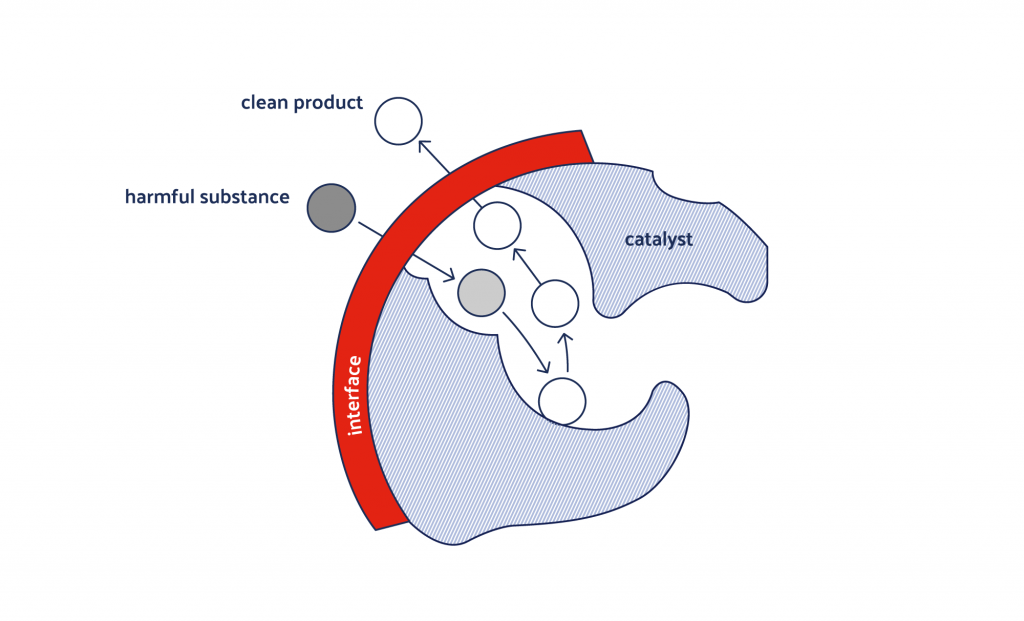
The activated, interface-free reaction zone of OxyCat® consists of millions of microelectrodes where the essential purification process takes place.
Redox Potential and Reactivity
Redox potential provides information about the oxidation power of the given redox couple. With the redox-active OxyCat® we can model the natural purification process under aerobic conditions prevalent in drinking and process water.
Because the redox potential of OxyCat® is high enough!
However, this alone does not give any indication about reaction speed. The reaction speed needed in order to meet the practical requirements when using reduced amounts of chemicals or natural oxygen is only reached at the ROS intermediate created by the microelectrodes.
Strong Results
Thus, the required disinfection can be reached within seconds thanks to the combination of high redox potential with highly reactive intermediates. With a significant reduction of chemical consumption.
Current internal studies suggest that microelectrode reactivity may be increased when adding an external electric power source. With that, we expect another increase in effectiveness.
Oxidation Catalyst – Development
The oxidation level on the silver surface is crucial for the catalytic effect of oxidation catalysts. This can be achieved by the use of heat up to several hundred degrees Celsius in an oxygen-rich atmosphere (see. Ekaterina Todadze, Ruhr-University Bochum, 2004).
Partial Oxidation
Thermal pre-treatment would, however, significantly impact activity at room temperature. We have therefore decided to perform partial oxidation of the silver surface in aqueous media at room temperature. This kind of oxidation using chemical oxidizing agents is comparatively easy. Activity is high for a short time, but then drops rapidly.
The catalysts were inadequate for practical use under these conditions. The main challenge was the stabilization of the „catalyst sandwich“. Our solution: A crystal-chemical assembly of a microporous semipermeable membrane made up of silver compounds that are thermodynamically stable.